
MBBS
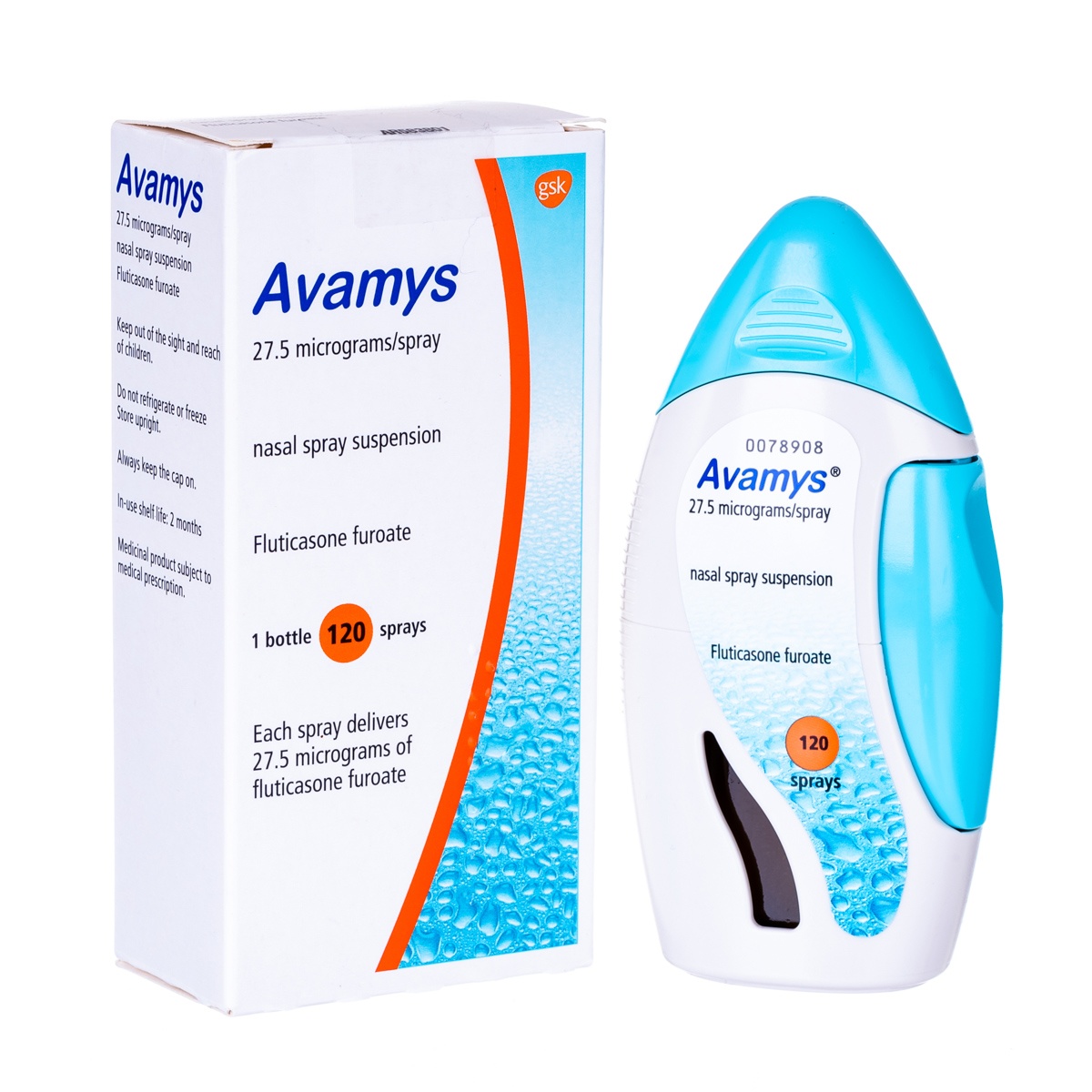
AVAMYS NASAL SPRAY. 27.5mg Spray 120 DOSES INHALER
- Does this requires a prescription ? Yes
- Generic Name: Fluticasone Propionate:27.5mg
- Manufactured by : Glaxosmithkline
- Drug Strength : 27.5mg
- Drug Form : Spray
- Pack Size : 120 DOSES INHALER
- Product SKU : dis_37509
Out of stock
Fluticasone Propionate:27.5mg | Inhalants
Manufactured / Marketed By : GSK Product Form : SprayPack Size : 120 DOSES INHALERIngredients : Fluticasone Propionate:27.5mgGeneric Category : Inhalants
How it works
AVAMYS NASAL SPRAY. 27.5mg Spray 120 DOSES INHALER is in a class of medications called corticosteroids. It works by blocking the release of certain natural substances that cause allergy symptoms.
Side effects
Major & minor side effects for AVAMYS NASAL SPRAY. 27.5mg Spray 120 DOSES INHALER
- White patches in the mouth or on the tongue
- Bone pain
- Diarrhea
- Fever
- Stomach pain
- Nausea and vomiting
- Sore throat
- Headache
- Cough
- Unusual tiredness and weakness
- Blurred vision
- Bloody nose
- Burning, itching, and irritation of the skin
- Skin rash
Uses of AVAMYS NASAL SPRAY. 27.5mg Spray 120 DOSES INHALER
What is it prescribed for?
-
Asthma - Maintenance
This medicine is used as a maintenance treatment of asthma, to prevent acute asthma attacks, in adults and children above 4 years of age.
-
Allergic rhinitis
This medicine is used to relieve the symptoms of allergic rhinitis such as sneezing, runny, stuffy, or itchy nose, etc.
-
Inflammatory skin conditions
The topical form of this medicine is used to treat inflammatory skin conditions such as dermatitis, eczema, atopic dermatitis, etc. Symptoms of these conditions may include redness of skin, skin blisters, soreness, itching, rashes, etc.
Concerns
Frequently asked questions
-
Onset of action
The amount of time taken by this medicine to show its effect is subject to vary based on the dose strength, the form of administration, and individual constitution.
-
Duration of Effect
The amount of time for which this medicine remains effective is subject to vary based on the dose strength, the form of administration, and individual constitution.
-
Safe with Alchohol?
Interaction with alcohol is unknown. It is advisable to consult your doctor before consumption.
-
Is it habit forming?
No habit-forming tendencies were reported.
-
Usage in pregnancy?
This medicine is not recommended for use in pregnant women unless absolutely necessary. All the risks and benefits should be discussed with the doctor before using this medicine.
-
Usage while breast-feeding?
This medicine is not recommended for use in breastfeeding women unless absolutely necessary. All the risks and benefits should be discussed with the doctor before using this medicine. If the medicine is used, the infant should be monitored closely for any undesired side effects.
When not to use?
-
Allergy
This medicine is not recommended for use in patients with a known allergy to AVAMYS NASAL SPRAY. 27.5mg Spray 120 DOSES INHALER , any other corticosteroids, or any other inactive ingredients present along with it.
-
Status asthmaticus
This medicine is not recommended for the primary treatment of asthma or other acute episodes of asthma where intensive measures are needed.
-
Nasal injury or Surgery
The nasal spray form of this medicine is not recommended for use in patients who have undergone a nasal surgery or have a nasal injury that is not completely healed.
Warnings
-
Pregnancy
This medicine is not recommended for use in pregnant women unless absolutely necessary. All the risks and benefits should be discussed with the doctor before using this medicine.
-
Breast-feeding
This medicine is not recommended for use in breastfeeding women unless absolutely necessary. All the risks and benefits should be discussed with the doctor before using this medicine. If the medicine is used, the infant should be monitored closely for any undesired side effects.
-
Growth retardation
This medicine may cause growth retardation in some children. Therefore, children receiving this medicine should be monitored closely with regard to growth parameters. The dose should be titrated to the minimum effective dose to reduce the chances of such side effects. Replacement with a suitable alternative may be necessary in some cases based on the clinical condition.
-
Visual disturbance
This medicine may cause blurred vision or other visual disturbances in some patients. Report any such symptoms to the doctor immediately. Appropriate corrective measures, dose adjustments, or replacement with a suitable alternative may be required based on the clinical condition of the patient.
-
Nosebleed and nasal ulceration
This medicine may cause nosebleeds and nasal ulcerations in some patients. Appropriate corrective measures, dose adjustments, or replacement with a suitable alternative may be required based on the clinical condition of the patient.
-
Candida infection
Patients using the systemic forms of this medicine for extended periods of time should be examined for candida infection or any other signs of adverse effects on the nasal and oral mucosa. Appropriate corrective measures, dose adjustments, or replacement with a suitable alternative may be required based on the clinical condition of the patient.
-
Impaired wound healing
The nasal spray form of this medicine is not recommended for use in patients who have experienced recent nasal trauma, nasal septal ulcers, nasal surgery, etc. until complete healing has occurred since this medicine may delay healing and worsen the patient's condition.
Dosage
-
Missed Dose
Inhaler/Nasal spray/Topical forms: Use/apply the missed dose as soon as you remember. If it is almost time for your next dose, skip the missed dose. Do not double your dose to make up for the missed one.
-
Overdose
Seek emergency medical treatment or contact the doctor in case of an overdose.
Interactions
Interaction with Medicines- Mifepristone severe
- Clarithromycin severe
- Ketoconazole severe
- Omacetaxine moderate
-
moderate
This medicine should be used with caution in patients having a high level of adrenal hormones in the body due to the increased risk of worsening of the patient's condition. Appropriate dose adjustments or replacement with a suitable alternative may be required based on the clinical condition of the patient.
-
moderate
This medicine should be used with extreme caution in patients with latent or active infections since this medicine can suppress the immune system and worsen the patient's condition. This risk is especially higher in patients on high doses of this medicine for a prolonged period of time. Appropriate dose adjustments or replacement with a suitable alternative may be required based on the clinical condition of the patient.
-
moderate
This medicine should be used with caution in patients with an ocular herpes infection due to the increased risk of worsening of the patient's condition. Appropriate dose adjustments or replacement with a suitable alternative may be required based on the clinical condition of the patient.
-
moderate
This medicine should be used with extreme caution in patients with a history of cataracts, glaucoma, or increased intraocular pressure due to the increased risk of worsening of the patient's condition. Appropriate dose adjustments or replacement with a suitable alternative may be required based on the clinical condition of the patient.
-
moderate
This medicine should be used with caution in patients with osteoporosis since this medicine may cause loss of bone density and further worsen the existing condition. Close monitoring of bone density, appropriate corrective measures, dose adjustments, or replacement with a suitable alternative may be required based on the clinical condition of the patient.
-
moderate
This medicine should be used with caution in patients with liver impairment due to the increased risk of severe adverse effects. Close monitoring of liver function may be required during treatment with this medicine. Appropriate dose adjustments or replacement with a suitable alternative may be necessary based on the clinical condition of the patient.
-
moderate
This medicine may alter the blood glucose levels in patients with diabetes. This interaction is based on the amount applied, the concentration of the medicine, and the size of the application area. Close monitoring of blood glucose levels is necessary for such patients during treatment with this medicine. Replacement with a suitable alternative may be required based on the clinical condition.
-
moderate
This medicine should not be applied to diaper rashes since the risks of systemic absorption and associated complications are significantly high. Replacement with a suitable alternative should be considered under your doctor's supervision.
General Instructions
Inhaler/Nasal spray: Use this medicine exactly as advised by your doctor. Do not use in larger or smaller amounts than advised/prescribed. Consult the doctor if you experience any undesirable side effects. Ensure that the treatment course is completed. Do not stop the use of this medicine without consulting your doctor. Topical forms: Apply a thin layer of the medicine to the affected area as instructed by the doctor. Follow all the usage instructions mentioned on the label or the package insert. Do not apply in larger or smaller quantities than recommended. Do not cover the application area with bandages or other coverings unless specifically instructed by the doctor. Contact your doctor if symptoms do not improve or resolve after 2 weeks of use.
Other details
- Usage does not depend on food timings
- To be taken as instructed by doctor
- Does not cause sleepiness
References
- medlineplus.gov - Fluticasone [Internet]. Available from: https://medlineplus.gov/druginfo/meds/a695002.html [cited 9/9/2020]. 2020
One of the following licensed pharmacy from the nearest location will deliver AVAMYS NASAL SPRAY. 27.5mg Spray 120 DOSES INHALER. The details of the licensed pharmacy shall be shared once you request the drugs and the respective pharmacy accepts your request based on valid prescription and availability.
Questions and Answers
There are no questions yet. Be the first to ask a question about this product.
Related products
Products listed on medicalstore.com.pk are 100% genuine and sourced from licensed pharmacies.
We work hard to bring you unimaginable discounts without compromising on quality.
We uses SSL (standard security technology) to ensure data privacy of all our customers.




4 reviews for AVAMYS NASAL SPRAY. 27.5mg Spray 120 DOSES INHALER
S H M AQEEL –
S H M AQEEL submitted 5 stars.
omer –
omer submitted 5 stars.
Fahad umer (verified owner) –
Fahad umer submitted 5 stars.
Muhammad Habib –
Muhammad Habib submitted 5 stars.